Abstract
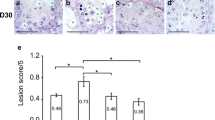
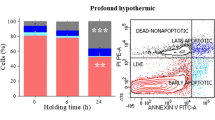
The objective of this study was to develop effective strategies for hypothermic preservation of immature porcine testis tissue to maintain structural integrity and cell viability. In Experiment 1, testes from 1-week-old piglets were used to study the effects of tissue sample size (as intact testes or fragments of 100-or 30 mg) and the use of one of 9 different media on hypothermic preservation of the testis tissue for 6 days. The examined media included: Dulbecco’s phosphate-buffered saline (DPBS), Dulbecco’s modified Eagle’s medium (DMEM), Leibovitz L15 (L15), L15 with fetal bovine serum (FBS, at 10%, 20% or 50%), HypoThermosol solution-FRS (HTS), Ham’s F12, and Media 199. On days 0, 3, and 6, testis tissues were digested to compare the cell survival rates. Tissue sections were also semi-quantitatively assessed to determine the efficiency of different preservation strategies. There was no effect of testis sample size (P > 0.05), but cell survival rates of testis cells isolated from preserved testis tissues changed depending on the media and day (P < 0.05). Testis tissue within HTS did not show morphological changes after 6 days. In Experiment 2, two of the top performing media (20% FBS-L15 and HTS) were selected for immunocytochemical detection of gonocytes. Proportions of gonocytes (%) in isolated testis cells, however, did not differ between the two media on days 0, 3, or 6. These results show that testis tissue can be maintained for 3 days at 4°C with high cell survival rate, and tissue morphology can be preserved for at least 6 days in HTS.





Similar content being viewed by others
References
Abrishami M, Anzar M, Yang Y, Honaramooz A (2010) Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology 73:86–96
Baicu SC, Taylor MJ (2002) Acid-base buffering in organ preservation solutions as a function of temperature: new parameters for comparing buffer capacity and efficiency. Cryobiology 45:33–48
Bonventre JV, Cheung JY (1985) Effects of metabolic acidosis on viability of cells exposed to anoxia. Am J Physiol Cell Physiol 249:C149–C159
Bronk SF, Gores GJ (1993) pH-dependent nonlysosomal proteolysis contributes to lethal anoxic injury of rat hepatocytes. Am J Physiol Gastrointest Liver Physiol 264:G744–G751
Crabbé E, Verheyen G, Tournaye H, Van Steirteghem A (1999) Freezing of testicular tissue as a minced suspension preserves sperm quality better than whole-biopsy freezing when glycerol is used as cryoprotectant. Int J Androl 22:43–48
de Rooij DG (1998) Stem cells in the testis. Int J Exp Pathol 79:67–80
Dobrinski I, Travis AJ (2007) Germ cell transplantation for the propagation of companion animals, non-domestic and endangered species. Reprod Fertil Dev 19:732–739
Ehmcke J, Schlatt S (2008) Animal models for fertility preservation in the male. Reproduction 136:717–723
Franca LR, Silva VA Jr, Chiarini-Garcia H, Garcia SK, Debeljuk L (2000) Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biol Reprod 63:1629–1636
Frankenhuis MT, Wensing CJG, Kremer J (1981) The influence of elevated testicular temperature and scrotal surgery on the number of gonocytes in the newborn pig. Int J Androl 4:105–110
Fuller BJ, Busza AL, Proctor E, Myles M, Gadian DG, Hobbs KEF (1988) Control of pH during hypothermic liver storage. Role of the storage solution. Transplantation 45:239–241
Goel S, Sugimoto M, Minami N, Yamada M, Kume S, Imai H (2007) Identification, isolation, and in vitro culture of porcine gonocytes. Biol Reprod 77:127–137
Goossens E, Frederickx V, Geens M, De Block G, Tournaye H (2008) Cryosurvival and spermatogenesis after allografting prepubertal mouse tissue: comparison of two cryopreservation protocols. Fertil Steril 89:725–727
Hochachka PW, Mommsen TP (1983) Protons and anaerobiosis. Science 219:1391–1397
Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S (2002a) Sperm from neonatal mammalian testes grafted in mice. Nature 418:778–781
Honaramooz A, Megee SO, Dobrinski I (2002b) Germ cell transplantation in pigs. Biol Reprod 66:21–28
Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I (2003) Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod 69:1260–1264
Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I (2004) Accelerated maturation of primate testis by xenografting into mice. Biol Reprod 70:1500–1503
Honaramooz A, Megee S, Zeng W, Destrempes MM, Overton SA, Luo J, Galantino-Homer H, Modelski M, Chen F, Blash S, Melican DT, Gavin WG, Ayres S, Yang F, Wang PJ, Echelard Y, Dobrinski I (2008) Adeno-associated virus (AAV)-mediated transduction of male germ line stem cells results in transgene transmission after germ cell transplantation. FASEB J 22:374–382
Hughes PE, Varley MA (1980) Reproduction in the pig. Butterworth, London
Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S (2007) Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod 22:1060–1067
Keros V, Hultenby K, Borgstrom B, Fridstrom M, Jahnukainen K, Hovatta O (2007) Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod 22:1384–1395
Lindell S, Nobel M, Rankin M, D'Alessandro A, Southard JH (1998) Optimal pH for simple cold storage or machine perfusion of dog kidneys with UW solution. Transplant Int 11:208–211
Milazzo JP, Vaudreuil L, Cauliez B, Gruel E, Masse L, Mousset-Simeon N, Mace B, Rives N (2008) Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum Reprod 23:17–28
Oatley JM, Brinster RL (2008) Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol 24:263–286
Ryu BY, Orwig KE, Kubota H, Avarbock MR, Brinster RL (2004) Phenotypic and functional characteristics of spermatogonial stem cells in rats. Dev Biol 274:158–170
Sethu P, Anahtar M, Moldawer LL, Tompkins RG, Toner M (2004) Continuous flow microfluidic device for rapid erythrocyte lysis. Anal Chem 76:6247–6253
Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, Kurome M, Nagashima H, Toyokuni S, Kogishi K, Honjo T, Ogura A (2002) Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod 17:3039–3045
Smith K, Garman L, Wrammert J, Zheng N-Y, Capra JD, Ahmed R, Wilson PC (2009) Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protocol 4:372–384
Song Y, Silversides FG (2007) Production of offspring from cryopreserved chicken testicular tissue. Poult Sci 86:1390–1396
Southard JH, Belzer FO (1995) Organ preservation. Annu Rev Med 46:235–247
Wyns C, Curaba M, Martinez-Madrid B, Van Langendonckt A, Francois-Xavier W, Donnez J (2007) Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum Reprod 22:1603–1611
Yang Y, Honaramooz A (2010) Effects of medium and hypothermic temperatures on preservation of isolated porcine testis cells. Reprod Fertil Dev 22:523–532
Zeng W, Snedaker AK, Megee S, Rathi R, Chen F, Honaramooz A, Dobrinski I (2009) Preservation and transplantation of porcine testis tissue. Reprod Fertil Dev 21:489–497
Acknowledgements
We thank Brian Andries and his staff, especially Margot Meiklejohn, at the Prairie Swine Center, and Dr. Murray Woodbury for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Portions of this study were presented at the 42nd annual meeting of the Society for the Study of Reproduction.
We thank the University of Saskatchewan Colleges of Graduate Studies and Veterinary Medicine for scholarships (to Y. Yang), and the Natural Sciences and Engineering Research Council (NSERC) of Canada for a summer student scholarship (to J. Steeg) and grants (to A. Honaramooz) to support this project.
Rights and permissions
About this article
Cite this article
Yang, Y., Steeg, J. & Honaramooz, A. The effects of tissue sample size and media on short-term hypothermic preservation of porcine testis tissue. Cell Tissue Res 340, 397–406 (2010). https://doi.org/10.1007/s00441-010-0946-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-010-0946-z